Neora sued by former Nerium supplier for false product testimonials
 Neora’s former product supplier, Nerium Biotechnology and Nerium Skincare (together represented as Nerium Biotech), have sued the company for allegedly using false product testimonials.
Neora’s former product supplier, Nerium Biotechnology and Nerium Skincare (together represented as Nerium Biotech), have sued the company for allegedly using false product testimonials.
In a July 23rd lawsuit filed in the Northern District of Texas, Nerium Biotechnology claims Neora is
using customer testimonials and “before and after” photographs of customers who used Biotech’s Nerium brand products to falsely advertise its new products and stifle competition.
Nerium Biotechnology’s dispute with Neora (then Nerium International), date back to a 2016 contract dispute lawsuit.
That lawsuit resulted in a June 2018 settlement, of which only some of the terms were ever made public.
In addition to what we’ve already reported regarding the settlement, Nerium Biotechnology has revealed the agreement required Olson to
- change the name of Nerium International ‘to something not “confusingly similar” to Nerium‘;
- stop using the word Nerium in its business operations;
- never refer to Neora as a successor to Nerium International;
- stop selling products manufactured by Nerium Biotech that used its patented “Nerium oleander extraction process”;
- release all trademarks and website domains used to sell Nerium Biotech products;
- turn over all copyrighted and copyrightable materials pertaining to Nerium Biotech’s products;
- make Neora’s distributors aware of the above restrictions and enforce their compliance.
Further discussing the settlement, Nerium Biotech states dispute that lead to the initial lawsuit
was one of Olson’s creation, as he had diverted tens of millions of dollars into his own pocket and was already in the process of improperly leveraging the NERIUM brand (which always belonged to Biotech) to peddle products that did not have the same active ingredient as the Nerium oleander products.
Caught red-handed, Olson agreed to pay Biotech $10 million and to give up International’s name, history, star product, and the goodwill associated therewith.
Nerium Biotech alleges that, by using testimonials pertaining to Nerium Biotech’s products, Olson has violated the settlement agreement.
Instead of a clean break from “Nerium,” Olson “rebranded” International to a confusingly similar-sounding name, “Neora,” hoping to forever intertwine the company with Biotech’s highly successful NERIUM Products.
Instead of relying on real reviews of real Neora customers who actually used its new products, Neora put out false ads using recycled video footage of International’s customers’ reviews of NERIUM Products and passed them off as its own.
Instead of relying on “before and after” photographs of Neora customers using its new products, Neora simply reused old “before and after” photographs of International’s customers who used NERIUM Products.
Instead of relying on its own results and being honest with the world about when its products came on the market, Neora put back-dated entries on its marketing blog and doctored old newspaper headlines to falsely make it appear as though it has been selling its new products years before the products were being sold.
Ironically, many of these false statements are found on Neora’s website promoting its ad campaign, “Real People, Real Results.”
Nerium Biotech claims Neora is intentionally deceiving the public and “causing significant confusion the marketplace”.
And not only is the company doing it, so too are Neora’s distributors.
Neora’s Brand Partners continue to conflate Neora’s new products with Biotech’s NERIUM Products.
That confusion is causing, and if left unrestrained will continue to cause, irreparable harm to Biotech.
Neora’s use of Nerium Biotech’s products expired in May 2019. The company claims it is about to “reintroduce Nerium brand products” into the marketplace.
Nerium Biotech also claims Neora and Olson have thus far failed to turn over copies of copyrighted and copyrightable marketing materials, as required by the agreed upon settlement.
Nerium Biotech claims the reason Neora has failed to turn over the material is because
Neora is now using those materials, and other false advertisements, to deceive the public into thinking that customers of Biotech’s NERIUM Products obtained their results using Neora’s new products.
One of the new products Nerium Biotech cites is “Neora Firm – Body Contour Cream”, which is a direct competitor to their “NeriumFirm – Body Contouring Cream” product.
Again in violation of the settlement agreement, Nerium Biotech cites the following as evidence of Neora representing its new products are a continuation of Nerium Biotech’s previously sold products.
People are happy with an iPhone 7 until the iPhone 10 is released with upgrades and new technology, then everyone wants the new and improved version.
The same is true for skincare technology, you must constantly be upgrading your science.
Instead of taking the easy road and resting on their laurels, Neora strives to improve their products when science and nature offer an authentic opportunity to make them even more powerful and effective.
Age IQ Night and Day Creams are the perfect example of seizing the opportunity to bring customers an even better product.
Jeff Olson purportedly made the above comments on May 31st, 2019.
Answers Nerium Biotech to Olson’s representation;
The problem for Neora (and for the public) is that Neora’s new products are not “new and improved version[s]” of the products that resulted in the “before and after” photographs and customer testimonials in its advertising.
Unlike the NERIUM Products, none of Neora’s new products use NAE-8® (Nerium Aloe Extract) as their active ingredient.
Yet, Neora continues to use customer testimonials and “before and after” photographs of customers who used NERIUM Products to sell its new, non-NAE-8® products.
Neora’s actions are false and deceptive.
Specific examples provided by Nerium Biotech include testimonials in a February 2019 Neora Firm marketing video:
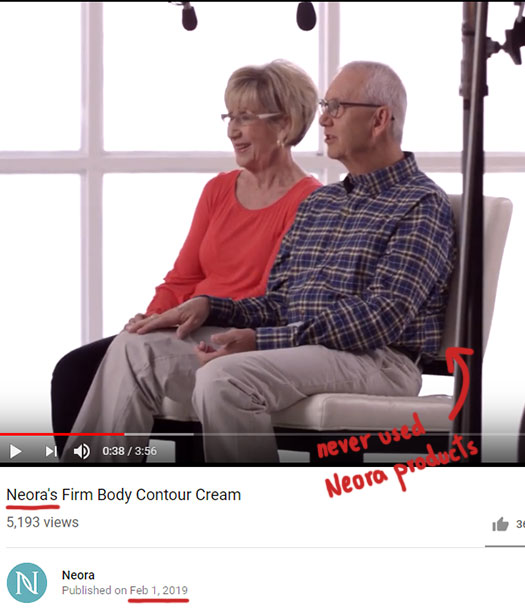
Which as below are taken from a 2015 Nerium Firm testimonial reel:
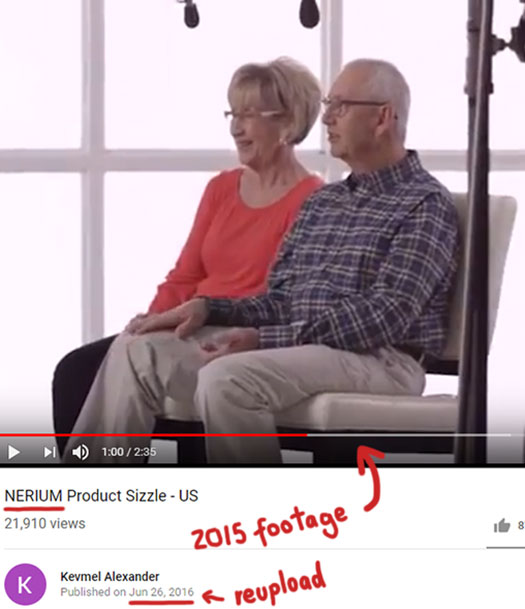
Footage featuring Olson himself talking about Nerium products also appears to have been recycled.
As have
- before and after photos from Neora’s filter feature on their website; and
- entries on Neora’s official blog (backdated to 2017 because they were copy and pasted from the now defunct official Nerium blog);
Examples of deception include:
- the changing of a Forbes article headline to a quote by Amber Olson to promote the article on Neora’s website;
- placing Neora product alongside independent media articles that do note feature Neora’s products; and
- Neora distributors using false before and after images on Instagram.
In an attempt to stop Neora, Nerium Biotech is seeking an injunction.
It is now evident that Neora never intended to adhere to its obligations under the IP Agreement and compete fairly with Biotech. Neora and Olson apparently see Biotech’s distribution of Nerium oleander skin care products as a direct threat to Neora’s new line of non-Nerium oleander products.
As per the settlement agreement, any disputes arising as a result of the agreement are to be mediated through arbitration.
Cited claims of relied include
- violations of the Lanham Act (false advertising); and
- breach of contract
If the requested injunction is granted, Neora will be required to comply with the settlement agreement – namely removing fraudulent advertising and making a public statement to clarify any caused confusion.
Stay tuned…
Update 10th September 2019 – Following Neora’s the removal of the identified offending material, Nerium Biotech has dismissed its lawsuit.


Thank you to all the readers who sent this one in today. Much appreciated.
Well, I’m confused. I’d like to know if Nerium Biotech is connected to MLM in some other way. I get they contracted with Olson’s MLM to provide oleander products aka Nerium International.
I now go on Nerium Biotech website and read this:
“As a Nerium distributor, you can simply buy at wholesale price then resale to your clients and patients at retail. This is no Multi-Level Marketing (MLM), here are some other benefits of working with us as a Nerium distributor.”
neriumrx.com/become-a-distributor/
But I call and she tells me it’s going to be available in the summer via MLM. Maybe they are going to have both options? If so, the price comparisons will be of great interest.
When I say connected to MLM, I mean the entire operation being questionable. We already know oleander is poisonous.
Not a bit surprised by any of this. Neora reps are telling everyone that the products are new and improved–yet they are completely different.
And if they are so much better, why use old pictures? Slimy snake.
How do I get the original Nerium Firm?
Nerium Biotech (and Nerium Skincare) aren’t MLM companies in and of themselves.
According to their complaint they’ll be selling the original Nerium range through Pure from August 1st.
MLM is not a bad way to make a good living. Olson took advantage of his brand partners.
The new company as I understand it will have no affiliation with Neora and will have the patent rights to the original product formulation.
I’ve used those products and know they work. I plan on joining the new company and make a good living at it.
I have some original Firm Pamela. I can help you get some.
Why not sign up now and buy wholesale per their website? Unless you don’t plan on doing a lot of product selling?
I can’t wait to get these products once again! I know they work as I used to be a consumer in the original Nerium back in 2013 – 2016.
So excited to get my hands on the Night Cream and Firm as well.
Nerium Biotech would haven’t had been successful with out Olsen!
It is so sad that so many people are hurt by the deception that is being created.
The good news is that the new company that has partnered with Nerium Biotech is a good company and I am so excited to be able to not only get the original Nerium products again but help get them into the hands of all the people that have been waiting.
Let’s just ignore their 3+ year history of this same garbage and just trust.. This time.. It will be different!!
LOLz!
Why not get them right now? Join for free, buy wholesale, use and actually sell them to real customers.
Seems something fishy is going on.
Article updated with lawsuit dismissal at Nerium Biotech’s request.
Anyone know the name of the new company that has partnered with Nerium Biotech?
It is Agellum.
(referral link removed)
This is my website if you want to see the new products by Nerium Biotech.