FTC sues Neora (Nerium) for being a pyramid scheme
 The FTC has filed a lawsuit against the MLM company Neora (formerly Nerium), and owner Jeff Olson.
The FTC has filed a lawsuit against the MLM company Neora (formerly Nerium), and owner Jeff Olson.
According to the FTC, Neora has operated as a pyramid scheme since inception as Nerium International in 2011.
Up until earlier this year Neora went by Nerium International. BehindMLM reviewed Nerium International back in 2014.
In our review we noted several autoship recruitment concerns;
Speaking of autoship, it conveniently brings us to the biggest red-flag I see in Nerium’s compensation plan.
For reasons not entirely clear to me, Nerium require affiliates to purchase a monthly 80 PV minimum autoship order or generate 200 PV in retail customer orders to qualify for commissions.
Shouldn’t this be the other way around?
The Fast Start Bonus for example, which is basically “sign up for autoship and recruit 3 affiliates who do the same”, perfectly illustrates potential problems.
We revisited Neora as Nerium and published an updated review in February.
To our dismay, in the five years since our original Nerium review, Neora failed to address its focus on affiliate autoship recruitment.
Following their own investigation, the FTC concluded;
Since its inception, Nerium has operated as an illegal pyramid scheme.
Unlike a legitimate multi-level marketing business, Nerium’s compensation scheme emphasizes recruiting new BPs over the sale of products to consumers outside of the organization.
Nerium’s business model makes it unlikely that BPs can earn money by selling product to outside consumers in response to genuine demand.
“Outside consumers” being retail customers, which are typically ignored in an affiliate autoship recruitment model.
With respect to retail sales, analysis of commission amounts reveals why Neora distributors (referred to as BPs) would opt for autoship recruitment over retail.
BPs who do not sign up for auto-delivery receive no discount on their product purchases, meaning that sales from their personal inventory would not be profitable even at full retail price.
Even for BPs who sign up for auto-delivery, the profit margin is typically slim or non-existent.
All in all, Neora had admitted to the FTC that
less than 1% of all rewards paid by the company consist of commissions paid on the sale of products to Retail Customers.
As is true in all pyramid schemes, the majority of Neora distributors made no money.
Here are the facts and figures quoted by the FTC pertaining to Neora distributor losses;
According to Nerium’s most recent reporting, less than 5% of BPs in the United States earn more from Nerium than they pay in fees and product purchases.
At least 92% of Nerium’s BPs have quit, with half leaving the company within six months or less.
The challenging reality of retailing and recruiting has meant that very few Nerium participants get above even the first rung on the organizational ladder.
Although there are now eighteen levels in the Nerium business opportunity hierarchy, Nerium reports that more than 85% of BPs have never reached the second rank.
Meanwhile, the overwhelming share of rewards accrues to the few individuals who reach the top ranks of Nerium’s organization.
And what little money Nerium distributors do make, are often clawed back in fees.
Nerium charges its BPs various fees which typically are far greater than any compensation they pay the BPs.
In particular, Nerium BPs have to pay out of their own pockets fees for sales aids, business cards, letterhead, and registration at multiple conferences, including Nerium’s annual multi-day “GetReal” conference.
Due to these numerous fees, according to Nerium’s most recent data, more than 95% of Nerium BPs paid more to Nerium each month than they earned in commissions and bonuses.
The FTC has also gone after Neora for deceptive marketing practices.
Nerium promotes its business by misrepresenting that BPs can earn a substantial income and achieve financial independence.
Despite promises of financial independence and six-figure incomes, less than 1% of “active” Nerium BPs averaged earnings of at least $530 in any given month.
A far smaller number earn that amount every month, which would still only result in an annual gross income of less than $6,400.
Needless to say nobody is achieving financial independence on $6400 annually or less.
 Co-defendants with Neora and Jeff Olson (right), are Signum Biosciences and Signum Nutralogix (together referred to as “Signum”).
Co-defendants with Neora and Jeff Olson (right), are Signum Biosciences and Signum Nutralogix (together referred to as “Signum”).
Signum, a merchant manufacturer, supplied Neora with their ME Sports and Nerium EHT products.
Signum launched EHT in 2014
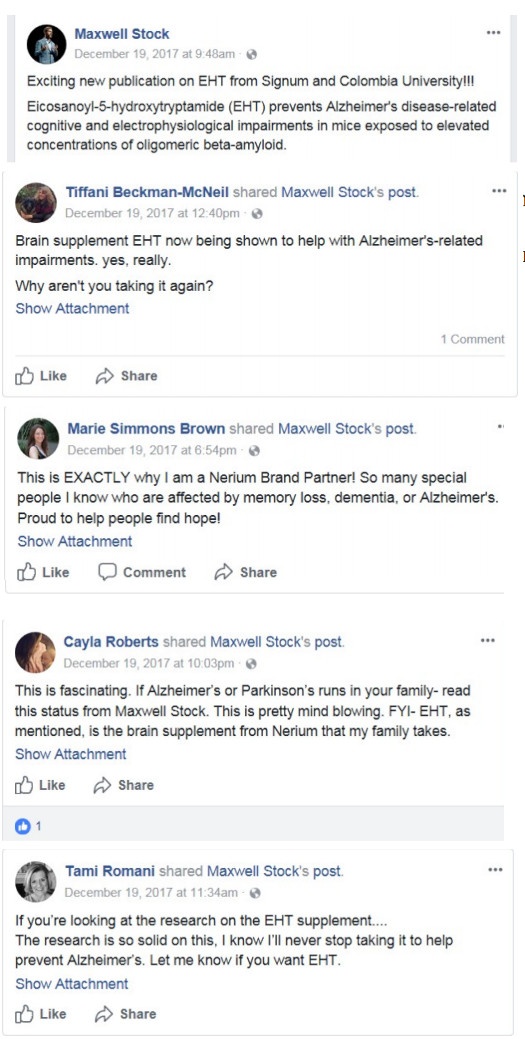
with input from Nerium’s marketing staff, conveying that EHT could help with CTE, Alzheimer’s disease, and Parkinson’s disease.
Defendants also have claimed that EHT is scientifically proven to offer users significant health benefits.
To date however, the only studies regarding EHT have involved rodents.
Defendants relied only on rodent studies, but they repeatedly conveyed to Nerium BPs who bought and sold Nerium EHT that these rodent studies were sufficient to establish the efficacy of EHT on humans.
Additionally, Defendants have falsely implied that Princeton University was involved in the development of EHT.
Defendants also have claimed that Signum has succeeded in developing medical breakthroughs relating to Alzheimer’s and Parkinson’s where actual pharmaceutical manufacturers have failed.
Signum initially approached pharmaceutical companies to distribute EHT.
The pharmaceutical companies approached insisted human trials be carried out, in order to validate EHT related marketing claims.
Rather than conduct these trials, Signum dumped EHT onto Nerium distributors.
Signum initially planned to proceed with clinical testing but ultimately rejected that approach as too costly.
Instead, Signum planned for Nerium’s network of hundreds of thousands of BPs—who were incentivized to recruit others to the scheme and generally had no scientific background—to distribute EHT Products and spread claims about it by “word of mouth.”
Neora’s (then Nerium) and Signum’s partnership began in 2014. For the most part, Neora handled Signum’s web presence, brand awareness and advertising campaigns.
Defendants formally announced their partnership and the launch of Nerium EHT at Nerium’s GetReal Conference for BPs in April 2015.
Presenting EHT to thousands of BPs at the April 2015 GetReal Conference, a Signum representative touted the significant funding Signum received from the Michael J. Fox Foundation and linked the claim that EHT protects and stabilizes proteins in the brain to the brain damage suffered by NFL player Junior Seau.
Immediately afterwards, Jeff Olson told the crowd, “a lot of things you can’t say, we’ll talk about that later on, because all those things you can’t say – it does!”
This pseudo-compliance lead to Nerium itself not providing information on Nerium EHT on their website.
In an April 2015 email, Nerium’s leading BP asked Defendant Jeff Olson and Nerium’s marketing director whether content from the ME Sports website would be copied into Nerium’s Digital Library, allowing BPs to easily provide that information to prospects.
Nerium’s marketing director responded that some of the content is only on Signum’s website “on purpose” because Nerium “cannot use/say [it] from reg[ulatory] standpoint.”
Defendants understood that even if Nerium purported to forbid certain disease claims, the Nerium BPs would still spread those claims in talking to potential customers and recruits.
In the end Nerium’s pseudo-compliance was for naught.
“The Michael J Fox Foundation for Parkinson’s research endorses EHT.” (Molly McConnell Dow, February 27, 2017);
“Do you know someone with Parkinson’s? Nerium EHT CAN HELP!” (Joanie Sullivan, June 15, 2016);
“Michael J Fox endorses EHT!” (Alex Forero, October 27, 2015);
“Do you or anyone you know suffer from Parkinson’s Disease? In a study published in 2013, EHT, the main ingredient in Nerium’s EHT supplement was tested and found to alleviate the symptoms of Parkinson’s.” (Skincaring.Nerium.com, December 17, 2015);
“Love my Princeton scientists. Listen to this video explaining EHT benefits…geared toward Parkinson’s. Alzheimer’s. Dementia.” (Amy E. Tate-Matthus, March 10, 2016).
“EHT for brain health . . . #alzheimers #dementia . . . #parkinsonsdisease #parkinsons.” (Carrie Skowronek Instagram, Sept. 1, 2019).
“Research and Funded by the Michael J. Fox Foundation . . . Had a concussion . . . family history of Alzheimer’s or Parkinson’s . . . Brain health is so important!!! #EHT.” (Maria Cipriano Instagram, October 10, 2019).
There are no human clinical trials showing that EHT prevents, reduces the risk of, or treats Parkinson’s disease, and there are no studies whatsoever, including human clinical trials, that have been conducted on EHT Products themselves related to that claim.
The FTC collected evidence of false medical claims regardless and sued Signum too.
The FTC accuses Neora, Jeff Olson and Signum of several FTC Act violations, including;
- running an illegal pyramid scheme (Nerium International and Jeff Olson);
- making false earning claims (Nerium International and Jeff Olson);
- making false or unsubstantiated efficacy claims (all defendants);
- making false establishment claims (all defendants);
- providing Neora distributors with the means and instrumentalities for the commission of deceptive acts and practices (all defendants)
The FTC alleges consumer injury as a result of Neora, Olson’s and Signum’s actions, and has asked for an injunction, disgorgement of ill-gotten gains and legal costs.
According to the November 1st press-release issued by the FTC, Signum has opted to settle the lawsuit.
At the time of publication the FTC’s lawsuit is yet to show up on Pacer. When it does, we’ll continue to track the case and provide you updates.
Update 2nd November 2019 – Neora has filed its own November 1st lawsuit against the FTC.
Update 11th September 2020 – Neora’s lawsuit against the FTC has been dismissed.
Update 11th October 2020 – The FTC’s case has been given a November 2022 trial date.
Update 29th September 2023 – On September 28th the Court ruled against the FTC, finding Neora wasn’t a pyramid scheme.


I’m aware Neora has filed a lawsuit of its own against the FTC. That’s coming up next.
Sad for the distributors and Signum too. Getting into business with Olson has turned out to be a terrible idea.
Neora is such a cult like environment it will be interesting to see what happens next.
My guess is that the distributors will stand by the company and see olson as a victim yet again. This lawsuit just barely scratches the surface of the shady things going on at that company.
When the FTC gets involved it’s usually a matter of time before the hammer drops on these companies.
Neora/Nerium claims that both they and the FTC have the receipts to prove that the companies retail customer volume is 61%.
thompsonburton.com/mlmattorney/2019/11/12/discussion-with-neora-co-ceo-deborah-heisz-regarding-ftc-litigation/
Makes you wonder why they went to court then hey…
I’ve heard the same from Neora corporate but am waiting to see how both cases play out.
Taking the FTC’s claims at face value in the meantime. Same with Neora’s claims.
Some people I know are in PURE, which I guess is a spinoff of Nerium?? Why does Nerium have so many co-companies.