Danelle Meoli misleads Awakend distributors on “exclusive rights”
![]() Awakend co-founder Danelle Meoli continues to mislead distributors and the public on “exclusive rights”, pertaining to the yet-to-be-shipped supplement Zenith.
Awakend co-founder Danelle Meoli continues to mislead distributors and the public on “exclusive rights”, pertaining to the yet-to-be-shipped supplement Zenith.
BehindMLM has been tracking the Zenith patent controversy since August.
The dispute, between TriPharma (now dba Vietal Nutrition) and Family First Business Ministry (FFBM), is scheduled for trial on March 28th, 2023.
Pending the outcome of the ongoing California lawsuit, the court noted the patent dispute remains “unsettled”.
That suit remains ongoing. The rights to the Patent remain unsettled.
Despite this, Meoli is telling Awakend distributors the company has “exclusive rights” to the disputed patent.
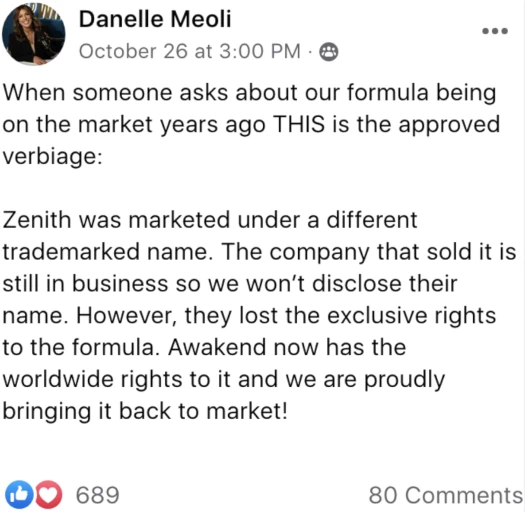
When someone asks about our formula being on the market years ago THIS is the approved verbiage:
Zenith was marketed under a different trademarked name.
The company that sold it is still in business so we won’t disclose their name.
However, they lost the exclusive rights to the formula.
Awakened now has the worldwide rights to it and we are proudly bringing it back to market!
When asked by an Awakend distributor about the court case referenced above, Meoli flat-out lied:
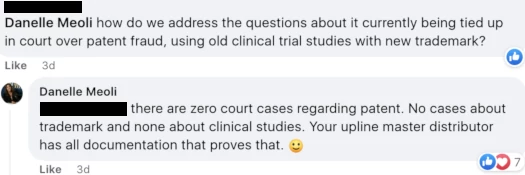
The disputed patent issue being unsettled isn’t my interpretation or opinion, I’ve provided that verbatim from a court order.
In the same private Awakend FaceBook group, another distributor pushed back on exclusivity;
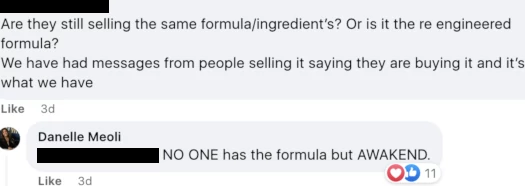
Meoli’s answer is again believed to be false. FFBM’s and Vietal Nutrition’s formulas are based on the same patent and ingredients (that’s why this is a legal issue).
Getting back to the patent dispute lawsuit, (TriPharma v. FFBM) et. al.), on October 20th BehindMLM reported that FFBM’s counterclaims against Awakend had been dismissed.
Four counterclaims were dismissed due to “newly discovered facts” being absent from the original filing. FFBM’s other two counterclaims were dismissed for being
so vague and conclusory that they fail to give rise to plausible claims against Tripharma.
The court granted FFBM permission to refile an amended counterclaim, which it did on October 26th.
In March 2011 FFBM paid millions of dollars to purchase all rights to the ’892 Patent, all rights to Trisynex, all rights to the UConn study and other clinical studies conducted on Trisynex, and all rights to the EJAP Publication.
FFBM has possessed the exclusive rights to the Trisynex product, the clinical studies, and the exclusive right to use and refer to the EJAP Publication (collectively referred to herein as “the Intellectual Property”) since October 1, 2014.
FFBM, however, has recently discovered that earlier this year the Tripharma Defendants falsely represented to the multi-level marketing company Awakend and its representatives that Tripharma had the exclusive rights to the Intellectual Property and that Tripharma could “assign” these rights to Awakend.
Through this fraudulent licensing arrangement, Awakend and the Awakend Individual Defendants are now falsely claiming it has the exclusive rights to the ’892 Patent (a patent that has EXPIRED) and the exclusive rights to the Intellectual Property, including the UConn Study and resulting EJAP Publication.
Awakend and the Awakend Individual Defendants have purported to “rebrand” the Trisynex product as Awakend’s “own product,” called Zenith.
As part of this “rebranding,” the Counter-Defendants have intentionally doctored the EJAP Publication to replace the tradename Trisynex (which was the actual subject of the UConn study) with “Zenith.”
BehindMLM covered Awakend’s doctored Zenith study in August. It has since been pulled from circulation.
FFBM brings this Counter-Complaint to pursue damages arising from Counter-Defendants’ fraudulent scheme, as well as injunctive relief to prevent Awakend from continuing to market, sell, or distribute its “Zenith” product and to stop Counter-Defendants from using or referring to the Intellectual Property and/or the ’092 Patent.
At this stage I don’t know if FFBM’s counterclaim is going to have an impact on the scheduled March 2023 jury trial. I’ll continue to monitor the case docket for updates.
Footnote: BehindMLM calling out Meoli’s misinformation shouldn’t be interpreted as “going in to bat” for FFBM.
BehindMLM has no stake in the outcome of this dispute, I’m just reporting on developments pending the final outcome of the case. We’ll cover the outcome regardless of whether TriPharma or FFBM prevails.
I maintain that Awakend should not have begun taking fees from consumers when the patent behind their flagship supplement (which for some reason still hasn’t shipped yet), remains in dispute.
We’d also like to see Meoli stop plying Awakend distributors with misinformation.
Update 20th January 2023 – The March 28th patent trial has been rescheduled to November 14th, 2023.


In related news, Awakend also rolled out its “founding member” NFTs last week:
Such value, much wow.
The Awakend label being shown on social media does not list the ingredients. This is in violation of FDA labeling law.
Danelle said it’s because it’s a proprietary blend so they don’t have to list them, which is false. Perhaps FDA will issue a recall once product ships.
I have been following this MLM with interest, mainly due to their egregious pricing of $160 per bottle.
A very fast Google search led me to a company selling TrimFit with Trysinex for $39.99 per bottle of 120 capsules with a Supplement Facts panel that clearly shows patents 6,899,892 and 10,632,092 called out on the label.
So, all is not as exclusive as it seems in Awakend diet pill land. Not sure if posting the vendor’s website is allowed by your site guidelines.
So reps are posting 1 million in sales in 1 week….
Distributors, no customers!
Product has shipped. Be ready for the amazing transformations (that include significant calorie reduction and hard work in the gym).
The new Zenith label is available. Interesting the ‘active’ ingredient is NOT Trisynex. It is a different blend called Celadrin – a blend of a patented combination of cetylated, esterified fatty acids containing cetyl myristoleate, cetyl myristate, cetyl palmitoleate, cetyl laureate, cetyl palmitate, and cetyl oleate extracted from plant-based source, with oils including palm, palm kernel, olive,nutmeg, coconut, and unsaturated vegetable oils.
The Clinical study was on TriSynex – and per the Health Canada filing that is 350 mg of cellulose, with 50 mg. cetlyated fatty acids.
The only clinicals I have found (so far) on Celadrin are for arthritis. Not leptin or weight loss.
Won’t they have to change their entire pitch now?
Ok. So looks like Lepitrim did have celadrin. So I amy be wrong.
I might be missing something but unless Trisynex is the active ingredient, then Zenith has nothing to do with the study and ongoing legal battle.
Which makes sense, as the court has reaffirmed the patent issue remains unresolved.
I’m not saying Celadrin doesn’t work – but it’s not what Awakend distributors and non-existent customers were pitched.
The formula in the original study was “modified cellulose and cetylated fatty acids”. Celadrin is cetylated fatty acids.
It is a proprietary blend, so maybe the trade secret is another fibre that is not listed on the label.
Labeling law requires every ingredient to be listed on the label.
You don’t have to reveal the amount of each in a proprietary blend, but you do need to list the ingredients. If they do not the FDA can issue a recall.
Following
Meloi comments on 10/28/22 regarding revised wording on trademark/patent claims, 2 days after she is a named defendant in a federal counterclaim.
Took a few days to spin out new verbiage? Always interesting times at Awakend.
Following.
Awakend is beginning to feel a lot like Elomir in that the claims are all over the place – withsome reps saying it helps them have more energy, better sleep, better focus, better mood.Yet nothing in the formula would do that.
For weight loss – some reps saying it is ‘instant’ results and posting photo shopped images (or images pushing their stomach out, then sucking it in) to show instant success. Others talk to the ‘8 week’ melt down as they refer to the clinical.
Customers are not seeing the miracle as fast. Several have begun to post they gained weight, had no weight loss, some said no weight loss but inches down.
Lot of complaints on bloating and gas – to the point where Danelle has told them to start with 2 capsules a day (which is not the clinical) and work their way up to 4 capsules a day as not everyone handles fiber well.
And they have turned off all commenting on posts in the customer page due, in my opinion, to the amount of gas comments that were being posted. Rep page comments have also been turned off.
Some reps claim you can eat whatever you want – no limits, and no changes. Some are saying you need to cut 500 calories a day and exercise.
The confusing part is the formula.In the Lepitrim study – it was 3 capsules of 450 mg (50 mg Celadrin, 350 viscous polysaccharides) before 2 meals (2700 mg a day spread over 2 meals). In the Beta test that Awakend did – it was take 2 capsules of 450 mg- 3 times a day (2700 mg a day spread over 3 meals).
I get fiber before a meal can make you fuller – but why change from the clinical? Was it to make sure 3 meals had fiber beforehand? In the Zentih label it is 1200 mg/2 capsules or 600 mg each before 2 meals (2400 mg a day spread over 2 meals).
In the Trisynex clinical it was 3 capsules of 400 mg each (50 mg fatty acids, 350 viscous polysaccharides).
Very confusing – does not appear the beta group or the Zenith label nor the Triysnex clinical match.
Celadrin lists viscous polysaccharides and then mystric and myristoleic acid. The Trisynex technical data sheet lists only mystric acid.
The formulas are similar (the patent lists many polysaccharides like carageenan, locust seed, etc) but different from what I see. Celadrin is in Zenith. Trisynex is not.
I have been taking Zenith for 4 weeks. I have gained weight and inches. The only thing I have noticed is I get fuller faster. No sleep improved like they claim.
None of my posts are accepted and I get no explanation as to why they are declined. I have now been blocked from Awakened Life group on FB twice.
I was stupid not to research this before I shelled out the money. Starting the process of getting refunded now before the 60 days come into play.
I jumped all in with a founders pack. I spent well over $3000. I too wished I would have researched better but I trusted a former “friend” from a past mlm company who contacted me to sign up and be on her team.
I have not lost weight. A few people that are on my team are not losing either.
I have a tiny bit to no of hope of getting some money back from there capsule dissolving issue.
I actually went looking online to see if there were any class actions filed on the company in hopes of a refund.
Don’t trust Meoli or the products. She jumps ship in every business endeavor.
I too went all in after seeing countless Doctors and Personal Trainers talk about how this was the magic pill we were all looking for and the results people had from years ago from the same formula.
I bought the lie hook, line and sinker and now I have a bunch of friends angry and still fat.
The company should do the right thing and issue refunds. If there’s a lawsuit to get just that, the amount paid, back, I’m all in!
HOW can we sue her and the company forthe 5K they stole from all of the founding members?
Legal questions should be directed to a lawyer.